Abstract
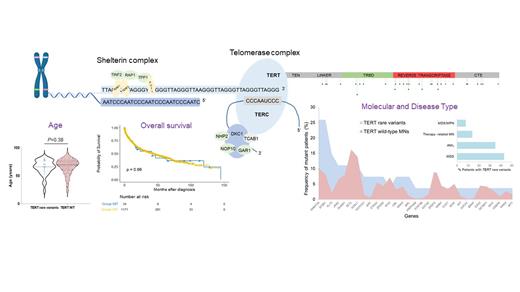
Dyskeratosis congenita is a prototypic inherited telomeropathy. Telomere length (TL) shortening has also been described in aplastic anemia (AA) and attributed in occasional patients to the presence of mostly heterozygous germline alterations in telomerase machinery genes (most commonly in the reverse transcriptase gene, TERT). Among various heterozygous variants described, certain TERT variants associated with shortened TL (e.g. H412Y and A202T) showed later a higher prevalence in general population questioning their pathogenicity and role as risk alleles. Nevertheless, shortened TL has been shown to correlate with poor outcomes in AA and myelodysplastic syndromes (MDS) along with a higher progression rate to acute myeloid leukemia, and increased non-relapse mortality after HSCT. A high prevalence (41/1514; 2.7%) of non-recurrent germline TERT rare variants, has been found in patients with MDS undergoing HSCT (Reilly et al Blood 2021) without any clinically apparent diagnosis of a telomere biology disorder. Despite their classification as VUS, these alterations were shown to result in impairment of telomere elongation in vitro and reduced TL in vivo. Clinically, VUS carriers were also characterized by younger age at MDS presentation (median 52 years) and shorter survival due to a higher incidence of non-relapse mortality.
We hypothesized that should such rare variants (traditionally thought as VUS) represent founder lesions and constitute risk alleles, one would expect to find some recurrence of these lesions in other cohorts of patients with myeloid neoplasia (MN). To test this hypothesis we screened a large meta-analytic cohort of MN patients (n=2560) including our patients (The Cleveland Clinic Foundation and MLL-Munich Leukemia laboratory) and a public series (The BEAT AML Master Trial and TCGA among others) for the presence of TERT coding variants. When whole genome sequencing was available (n=1432) TelomereHunter tool was used to estimate TL in carriers of TERT rare variants and wild-type (WT) cohorts.
Overall, we identified 73 TERT coding variants. Based on maximum gnomAD population allele frequency (AF) <0.001 and ACMG and pathogenicity scores according to the classifiers of VarSome (https://varsome.com), we found 37/2560 (1.4%) variants which were categorized as rare and VUS. Of note, only 6 patients harbored established TERT pathogenic variants while the rest were common and VUS. Alterations occurred in the RTD (52%), TRBD-Linker region (29%) and CTE domain (19%). When compared to the aforementioned HSCT cohort described by Reilly et al, TERT rare VUS had a lower frequency and did not overlap with any of the VUS reported in this study, except for 2 patients harboring one variant each in amino acid locations 741 and 1041. Assessment of TL, possible in 12 patients carrying TERT rare variants, did not reveal differences compared to TERT WT cases. Interestingly, none of these rare variants were also observed in a control cohort of patients with AA (n=268).
In terms of clinical features, MN patients with TERT rare variants harbored more somatic hits in DNMT3A and SF3B1 (26% each), and FLT3 (15%) with a diverse spectrum of additional molecular lesions, including germline VUS of other genes (4 patients harbored concomitant VUS in BRCA1, FANCG, and SAMD9, with another patient carrying a likely benign SAMD9L variant) (Fig1). When compared to WT counterparts, patients harboring TERT rare variants had a similar median age at MN diagnosis (70 vs. 66, P= 0.38) and similar survival outcomes (P= 0.96). Importantly, HSCT outcomes were ascertained only in 7 cases because of the real-life nature of our cohort which compiles MN patients of any subtype and age, thereby not biased by transplant referral like in the aforementioned study focusing only on MDS patients undergone HSCT. Finally, principal causes of death were primary disease (63%), presence of another malignancy (19%) and infections (13%) and, of note, no patient died of non-infectious pulmonary causes.
In sum, our study, while confirming a relatively high (albeit not significant vs. controls) combined TERT VUS population burden in MN, also strongly indicates that rare TERT VUS are non-recurrent. Therefore, they cannot be considered risk alleles individually nor can their biological (combined or individual) impact in terms of non-transplant outcomes, irrespective of their in vitro functional effects.
Carraway: Celgene, a Bristol Myers Squibb company: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Stemline: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Agios: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Other: Independent review committee; Takeda: Other: Independent review committee; Astex: Other: Independent review committee; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Haferlach: MLL Munich Leukemia Laboratory: Other: Part ownership. Maciejewski: Bristol Myers Squibb/Celgene: Consultancy; Alexion: Consultancy; Novartis: Consultancy; Regeneron: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal